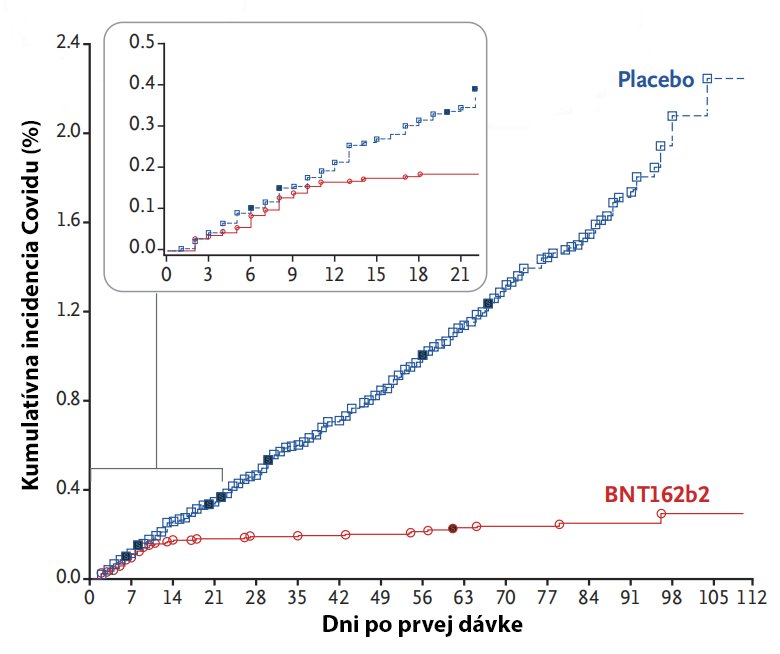
Ak niekto v súčasnosti hovorí, že očkovanie proti Covidu nie je účinné, respektíve že neexistujú dostatočné dôkazy o jeho účinnosti, tak si danú problematiku vôbec nenaštudoval.
3. fáza klinického skúšania Pfizer/BioNTech/BNT162b2 mRNA vakcíny (18) porovnávala 21720 osôb, ktorým bola aplikovaná vakcína, a 21728 osôb, ktorým bola aplikovaná injekcia s fyziologickým roztokom (placebo). 2 dávky vakcíny zabezpečili 95% ochranu proti symptomatickej infekcii (infekcia s príznakmi). Pozorované nežiaduce účinky boli vlastne znaky imunitnej rekacie podobne ako u iných vakcín (vyčerpanosť, bolesť hlavy, bolesť svalov a kĺbov, zimnica, …).
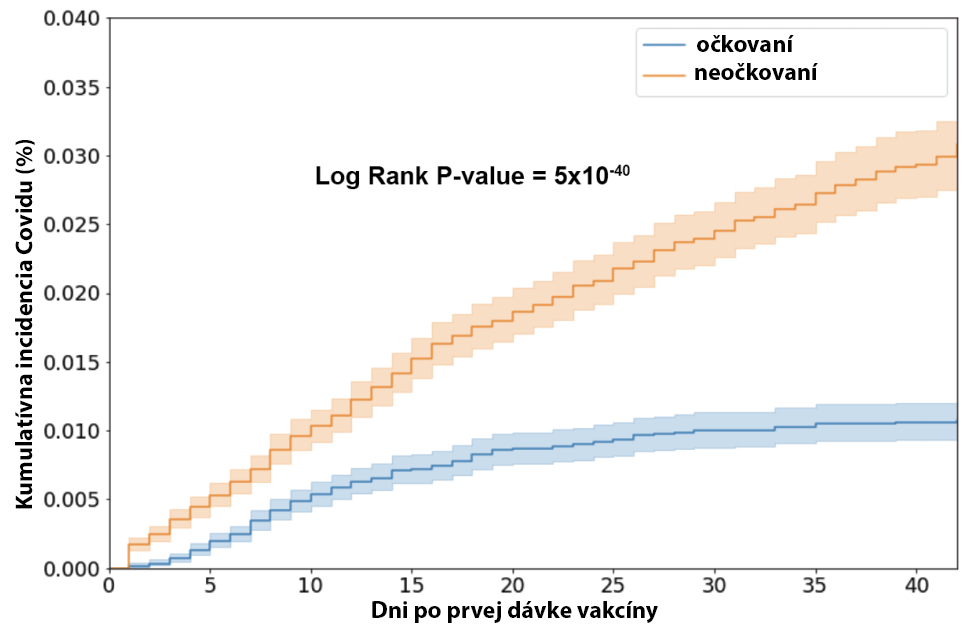
10 Nasledujúcich štúdií v tabuľke porovnávalo výskyt Covid infekcií, vrátane tých asymptomatických, u očkovaných a neočkovaných. Potvrdzujú vysokú účinnosť po očkovaní 2 dávkami Pfizer/BioNTech vakcíny.
| štúdia | počet osôb | účinnosť 2 dávok proti infekcii | poznámka |
| Pawlowski (1) | 31069 očkovaných 31069 neočkovaných | 89% | |
| Swift (2) | 49220 | 97% | |
| Thompson (3) | 3975 | 91% | |
| Björk (4) | 805741 | 86% | |
| Lumley (5) | 13109 | 90% | |
| Hall (6) | 23324 | 85% | |
| Shah (7) | 144525 | 92% | zníženie šírenia nákazy o 60% |
| Heymann (8) | 6286 | 89% | |
| Dagan (9) | 596618 očkovaných 596618 neočkovaných | 92% | 90% účinnosť proti asymptom. infekcii |
| Moustsen-Helms (10) | 39040 seniori 331039 zdravotní pracovníci | 64% 90% |
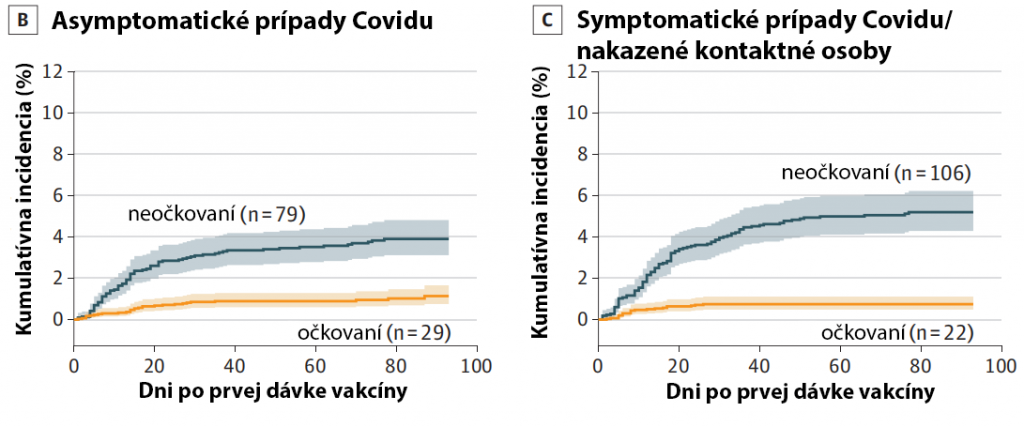
Výsledky ďalších 5 štúdií jasne ukazujú účinnosť Pfizer/BioNTech vakcíny proti asymptomatickej (bezpríznakovej) infekcii.
| štúdia | počet osôb | účinnosť 2 dávok proti asymptom. infekcii | poznámka |
| Tang (11) | 2776 očkovaných 2165 neočkovaných | 90% | |
| Haas (12) | 6538911 | 92% | |
| Angel (13) | 5372 očkovaných 696 neočkovaných | 86% | |
| Regev-Yochay (14) | 9650 | 65% | zníženie šírenia nákazy |
| Tande (15) | 39156 | 80% |
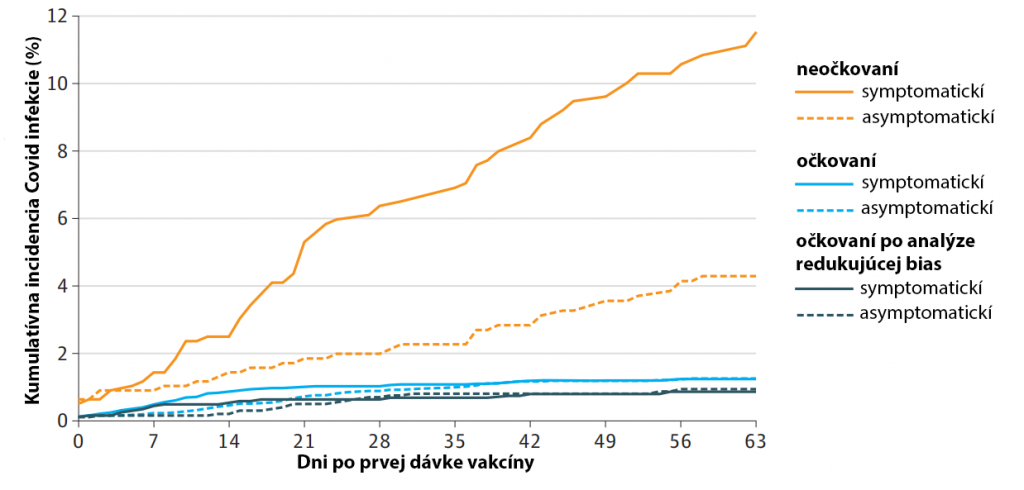
Pár poznámok na záver:
– očkovanie znižuje výskyt Covidu nielen u očkovaných, ale aj u neočkovaných prostredníctvom kolektívnej imunity (16, 17, 19, 24, 25, 26)
– žiadna vakcína nie je na 100% účinná podobne ako napr. ani bezpečnostné pásy v aute nie sú na 100% účinné, preto je pochopiteľné, že malé percento očkovaných stále môže ochorieť (s ľahším priebehom ako u neočkovaných), a je taktiež pochopiteľné, že to nijako nevyvracia výsledky vyššie uvedených štúdií
– ak sa očkovaní nakazia, majú menšiu vírovú nálož, a tak aj významne nižšiu pravdepodobnosť šírenia infekcie v porovnaní s neočkovanými (20, 21, 22, 23, 3, 14)
Článok bol naposledy aktualizovaný 24.7.2021.
Zdroje:
1.Pawlowski C LP, Puranik A, et. al. FDA-authorized COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. medRxiv. 2021; https://www.medrxiv.org/content/10.1101/2021.02.15.21251623v1.full.pdf
2. Swift MD, Breeher LE, Tande AJ, Tommaso CP, Hainy CM, Chu H, et al. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin Infect Dis. 2021.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8135611/pdf/ciab361.pdf
3. Thompson MG et al. Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. NEJM. 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2107058
4. Björk J. IM, Moghaddassi M., et al. Effectiveness of the BNT162b2 vaccine in preventing COVID-19 in the working age population – first results from a cohort study in Southern Sweden. medRxiv. 2021; https://www.medrxiv.org/content/10.1101/2021.04.20.21254636v1
5. Lumley SF RG, Costantindes B., et al. An observational cohort study on the incidence of SARS-CoV-2 infection and B.1.1.7 variant infection in healthcare workers by antibody and vaccination status medRxiv. 2021; https://www.medrxiv.org/content/10.1101/2021.03.09.21253218v1.full.pdf
6. Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725-35.https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00790-X/fulltext
7. Shah A GC, Bishop J, et al. Effect of vaccination on transmission of COVID-19: an observational study in healthcare workers and their households. medRxiv. 2021; https://www.medrxiv.org/content/10.1101/2021.03.11.21253275v1
8. Heymann AD. ZG, Shasha D., et. al. BNT162b2 Vaccine Effectiveness in Preventing Asymptomatic Infection with SARS-CoV-2 Virus: A Nationwide Historical Cohort Study. Lancet (preprint). 2021;https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3796868
9. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021.https://www.nejm.org/doi/full/10.1056/nejmoa2101765
10. Moustsen-Helms I EH, Nielsen J, et. al. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 Vaccine in long-term care facility residents and healthcare workers – a Danish cohort study medRxiv. 2021; https://www.medrxiv.org/content/10.1101/2021.03.08.21252200v1.full.pdf
11. Tang L, Hijano DR, Gaur AH, Geiger TL, Neufeld EJ, Hoffman JM, et al. Asymptomatic and Symptomatic SARS-CoV-2 Infections After BNT162b2 Vaccination in a Routinely Screened Workforce. JAMA. 2021.https://jamanetwork.com/journals/jama/fullarticle/2779854
12. Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021.https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00947-8/fulltext
13. Angel Y. SA, Henig O., et al. Association Between Vaccination With BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers. JAMA (preprint). 2021;https://pubmed.ncbi.nlm.nih.gov/33956048/
14. Regev-Yochay G AS, Bergwerk M, et al. Decreased Infectivity Following BNT162b2 Vaccination. Lancet (preprint). 2021; https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3815668
15. Tande AJ, Pollock BD, Shah ND, Farrugia G, Virk A, Swift M, et al. Impact of the COVID-19 Vaccine on Asymptomatic Infection Among Patients Undergoing Pre-Procedural COVID-19 Molecular Screening. Clin Infect Dis. 2021.https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab229/6167855
16. Milman O et al. Community-level evidence for SARS-CoV-2 vaccine protection of unvaccinated individuals. Nature Medicine. 2021. https://www.nature.com/articles/s41591-021-01407-5
17. Salo J et al. The indirect effect of mRNA-based Covid-19 vaccination on unvaccinated household members. medRxiv. 2021.https://www.medrxiv.org/content/10.1101/2021.05.27.21257896v1
18. Polack FP et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020.https://www.nejm.org/doi/full/10.1056/nejmoa2034577
19.Harris RJ et al. Effect of vaccination on household transmission of SARS-COV-2 in England. NEJM. 2021. https://www.nejm.org/doi/full/10.1056/NEJMc2107717
20.McEllistrem MC et al. Single dose of a mRNA SARS-CoV-2 vaccine is associated with lower nasopharyngeal viral load among nursing home residents with asymptomatic COVID-19. Clinical Infectious Diseases. 2021. https://academic.oup.com/cid/advancearticle/doi/10.1093/cid/ciab263/6188727
21.Bailly B et al. BNT162b2 Messenger RNA Vaccination Did Not Prevent an Outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 Variant 501Y. V2 in an Elderly Nursing Home but Reduced Transmission and Disease Severity. Clinical Infectious Diseases. 2021. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab446/6276392
22.Levine-Tiefenbrun M et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nature Medicine. 2021. https://www.nature.com/articles/s41591-021-01316-7
23.Petter E et al. Initial real world evidence for lower viral load of individuals who have been vaccinated by BNT162b2. medRxiv. 2021. https://www.medrxiv.org/content/10.1101/2021.02.08.21251329v1
24.Monge S. Direct and indirect effectiveness of mRNA vaccination against SARS-CoV-2 infection in long-term care facilities in Spain. medRxiv. 2021. https://www.medrxiv.org/content/10.1101/2021.04.08.21255055v2
25.Prunas O. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. medRxiv. 2021. https://www.medrxiv.org/content/10.1101/2021.07.13.21260393v1
26.Layan M et al. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study. medRxiv. 2021.https://www.medrxiv.org/content/10.1101/2021.07.12.21260377v1